mediSKIN
SEED/0719/0200
Development of novel medical-grade skincare products for oncology patients
Introducing mediSKIN, an innovative project supported by the European Union’s NextGenerationEU plan and the Republic of Cyprus through the RESTART 2016-2020 program.
With a primary focus on “Strengthening the Competitiveness of the Economy,” the Research and Innovation Foundation is supporting this initiative with a budget of 498,505 Euros.
mediSKIN aims to enhance the well-being and quality of life for cancer patients through cutting-edge advancements in medical-grade skincare.
Novelty of mediSKIN project
Novel medical-grade atelocollagen
developed by Promed Bioscience and characterized by CUT
Formulation, blending and prototyping of mediSKIN product
developed by RSL Revolutionary Labs
In vitro efficacy and safety testing in 3D skin models
tested and analyzed by Theramir
Clinical trials and assessment of patients’ quality of life
by GOC and CUT
mediSKIN PARTNERS

RSL Revolutionary Labs Ltd’s expertise in natural extracts and medicinal formulations will once again be utilized to develop super stable and highly effective molecularly engineered blends.
Our partner Promed Bioscience once again leads the way, with the development of not one but three novel types of high-grade atelocollagen to be incorporated in our unique formulas.
Theramir Ltd with significant experience in pharmaceutical preclinical testing, will be responsible for assessing the efficacy and safety of the novel formulas by using 3D engineered in vitro skin models.
Cyprus University of Technology‘s rigorous efforts will characterise this novel biomaterial using state-of-the-art techniques and in-house developed analysis methods.
The project activities will include the first Cyprus-based clinical trial, organised by German Oncology Center and CUT, to assess the effect of the mediSKIN-developed formulas on patient quality of life.
FUNDING AGENCIES
More about the project
PROBLEMS IN ONCOLOGY CARE
While advancements in current treatments have led to increased survival rates and improved quality of life for oncology patients, the presence of dermatological toxicities remains a significant concern. However, the available options for managing skincare in cancer patients are still relatively limited. Specifically designed products targeting patients who are experiencing dermatological side effects are not yet widely available, are often ineffective and may potentially contain harmful chemicals.
THE CHALLENGES
Oncology wounds
The presence of serious skin-related side effects frequently contributes to patients’ noncompliance with their treatment regime, while the options available for managing the health of the skin remain limited and ineffective.
Skin toxicities due to therapy | Malignant & Paliative care wounds | Other side effects
THE SOLUTION
The specific goal of the current study is the development of natural topical skincare products that utilize a novel water-soluble atelocollagen complex. This specially designed collagen formulation penetrates deep into the skin, offering relief from a range of dermatological issues commonly experienced by oncology patients and facilitating effective wound healing. The objective of the mediSKIN project is thus to generate innovative solutions that can successfully alleviate a range of skin conditions while promoting optimal healing of wounds and enhancing the overall skincare and well-being of patients undergoing oncology treatment.
Science and R&D
Novel collagen synthesis
Triple-helix atelocollagen (CollX3) was developed through a series of modifications resulting in a protein complex of three distinct MW.
In vitro biocompatibility
In vitro cell viability assessment in a range of human skin cell lines.
3D-skin assays
In vitro skin irritation test of CollX3 in reconstructed human skin epidermis and corneal epithelium.
Tumorigenicity
Tumorigenicity safety assessment on colorectal and ovarian cancer cells.
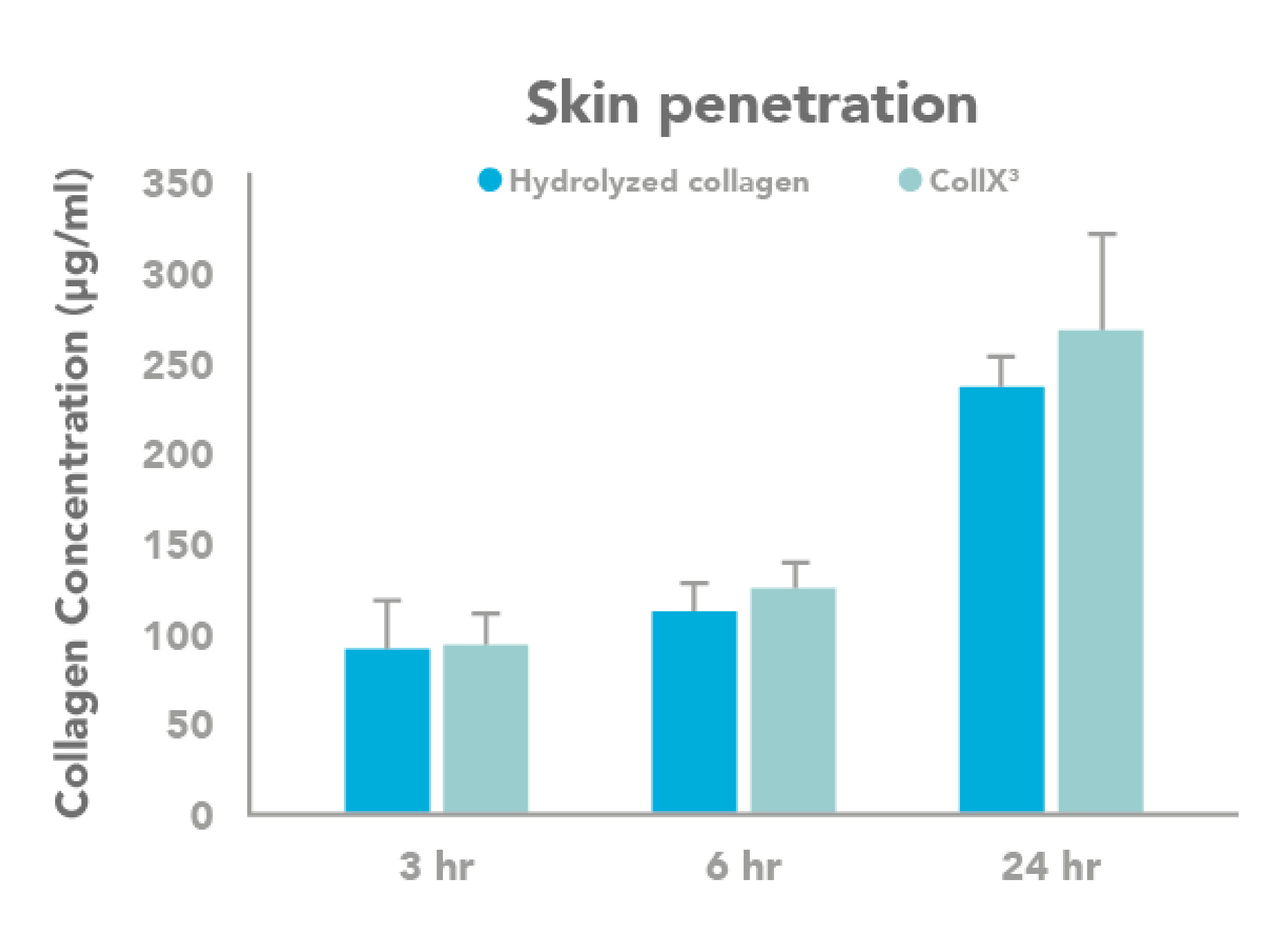
Penetration assessment
Ex vivo penetration assessment on human skin epidermis and dermis.
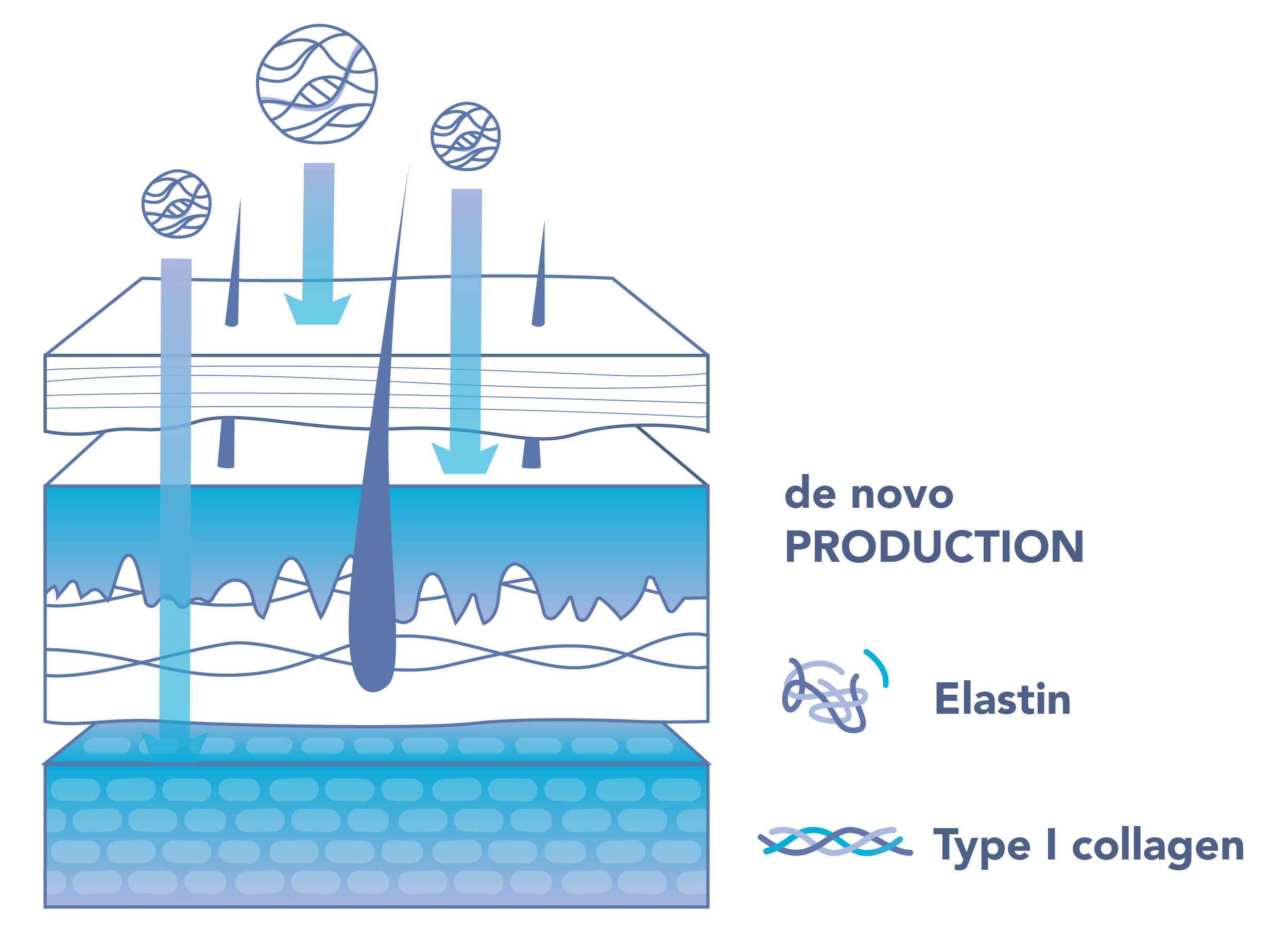
Assessment of regeneration markers
Expression levels of COL1 and ELN validated via qPCR in dermal human fibroblasts.
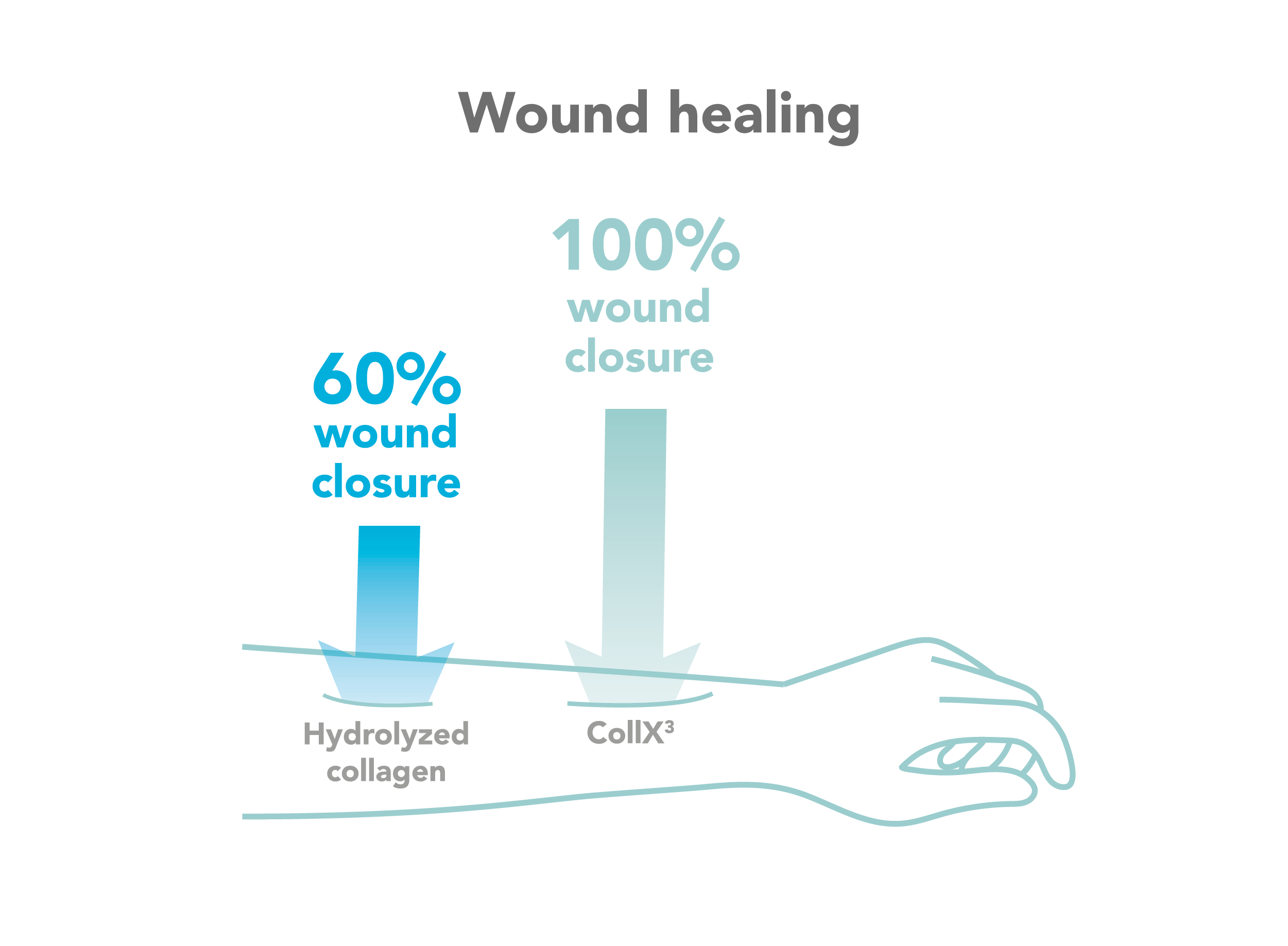
Wound healing
In vitro and ex vivo wound healing assessment on human epidermis and dermis tissues.
Research & development
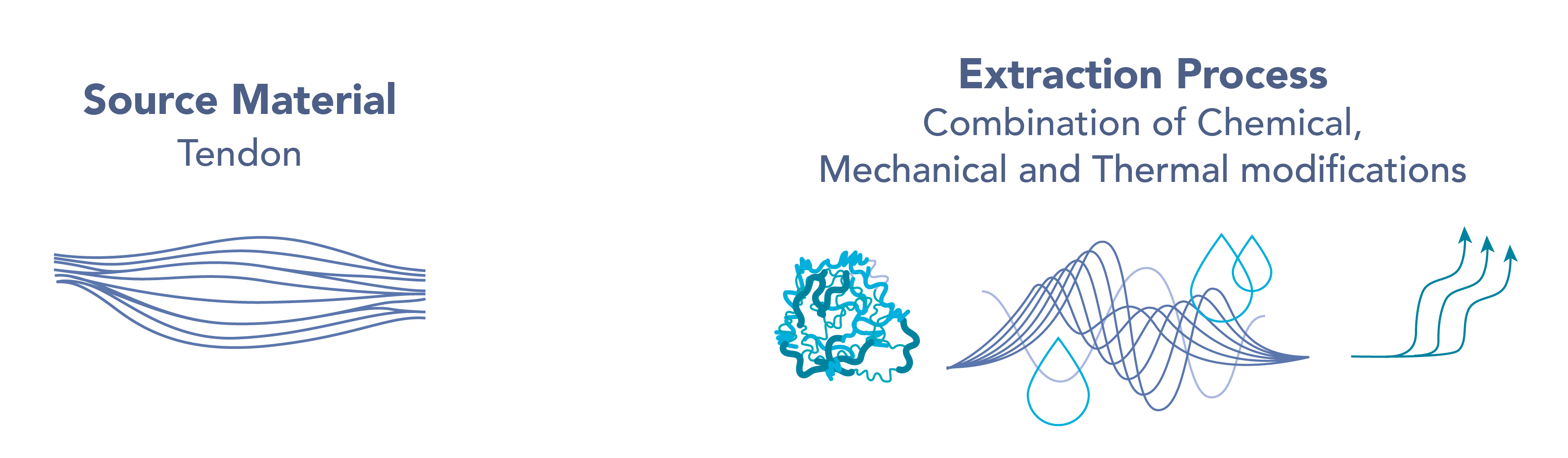
A unique atelocollagen complex was developed, containing three distinct molecular weight ranges intended to penetrate deep into the skin.
CollX3 features a triple-helix medical-grade atelocollagen, sourced from tendons, and extracted via a proprietary process that precisely cleaves the telopeptides located at the ends of the native collagen protein.

The benefits of having a wide range of molecular weights
| High molecular weight collagen (up to 120kDa) Provides structural support to the epidermis, increases thickness and improves stability. Enhances proliferative activity and cell turnover. |
|
| Medium molecular weight collagen (up to 60kDa) Stimulates wound-healing factors and promotes the production and organization of collagen matrix. Supports recovery of the skin. |
|
| Low molecular weight collagen (up to 20kDa) Promotes tissue regenerative effects, aids in the maturation of the skin after scarring and supports the natural healing process. |
Biocompatibility Assessment of CollX3
In vitro evaluation of CollX3 reveals outstanding biocompatibility, with no observed toxicity and no induction of cell death in human dermal fibroblast cells. Moreover, in vitro skin irritation test of CollX3 shows no signs of irritant activity or change in cell viability in reconstructed human skin epidermis and corneal epithelium. Additionally, tumorigenicity assessment demonstrates that CollX3 is non-carcinogenic, rendering it safe for dermaceutical applications.
Introducing
εὖSKIN ADVANCED
A uniquely designed skincare product that harnesses the power of the innovative atelocollagen complex CollX3. The specially formulated solution is designed to offer unparalleled support in the prevention and recovery of skin damage caused by radiotherapy, chemotherapy, or other side effects commonly associated with oncological treatments.
With its targeted formulation, εὖSKIN Advanced addresses the specific needs of patients undergoing treatment, while working to promote the healing and rejuvenation of the skin.
Clinical trials and assessment of patients’ quality of life
This topical formulation has been granted approval as a cosmetic-grade product by the Cyprus Pharmaceutical services of the Ministry of Health (File no 21.14.10 No of Cert: 07/2022 and European CPNP Portal notification number: 4091379).
A multicenter clinical trial is currently underway to assess the efficacy of mediSKIN and CollX3 in wound healing. The trial is actively recruiting participants in Cyprus and Greece (NCT05588973).
Clinical trials patients in Cyprus under radiotherapy treatment
Clinical trials patients in Greece under radiotherapy and chemotherapy treatments


mediSKIN seminars
The first seminar was held on 18.05.2022 by specialized doctors, psychological consultants and oncology researchers.
The seminars aim to inform oncology patients and health professionals.





















